- maintrac
-
Information for Patients
-
maintrac® in Cancer Diseases
maintrac® enables the gentle detection of living circulating tumor cells in the blood of patients with cancer and supports monitoring of disease progression as well as individualized therapy adjustment.
-
How effective is your therapy?
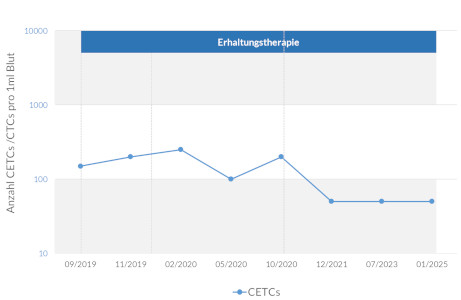
Monitoring changes in the number of living circulating tumor cells shows the effectiveness of the therapy and correlates with the further course of the cancer.
-
Which medications work best for you?
With the maintrac® drug sensitivity test, it can be examined how your circulating tumor cells respond to various chemotherapeutic agents – for a more targeted treatment.
-
Which maintenance therapy is appropriate for you?
maintrac® examines therapy-relevant characteristics of your tumor cells and helps to identify which maintenance therapy may be useful for you – individual and targeted.
-
Has my cancer spread?
With stemtrac®, especially aggressive tumor stem cells in the blood can be detected – a possible indication of metastasis and an increased risk of relapse.
-
maintrac® in Cancer Diseases
-
Information for Physicians
-
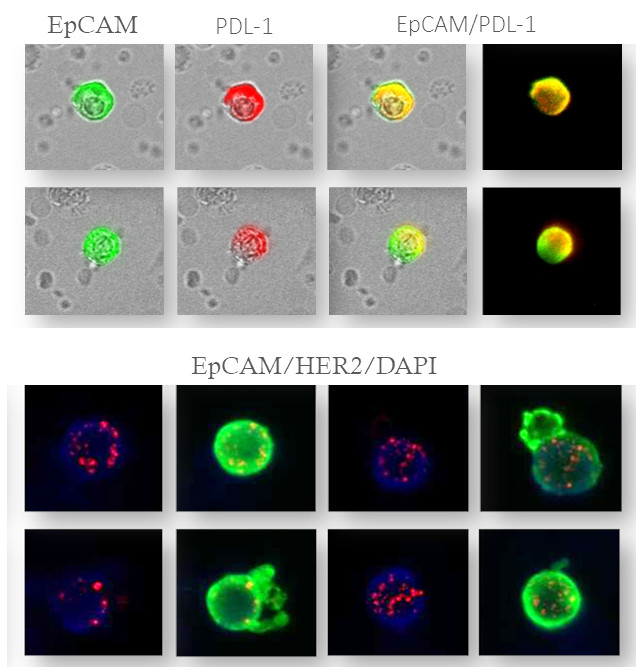
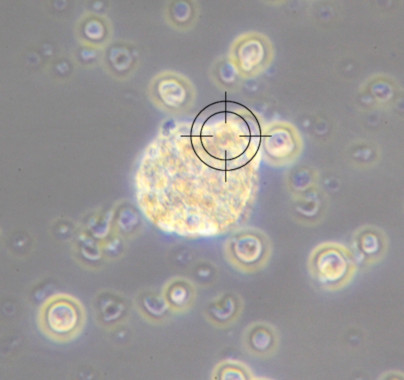
Cell Counting
maintrac® cell count enables the precise detection of viable circulating tumor cells (CETCs / CTCs) in the blood. The method allows for the quantitative tracking of these cells over time and provides insights into disease progression, therapy response, and also serves to monitor Minimal Residual Disease (MRD). It is suitable for monitoring disease course and supporting therapy decisions in cancer patients.
-
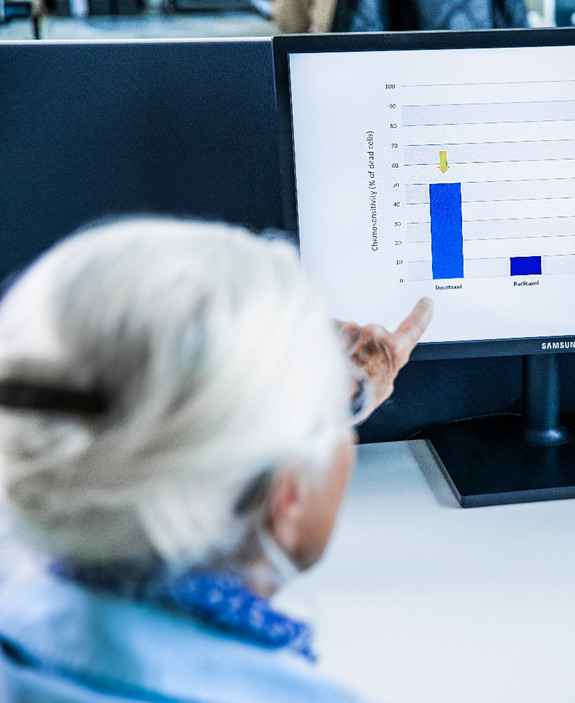
Drug Sensitivity Testing
maintrac® drug sensitivity testing evaluates in vitro the effect of various substances directly on viable circulating tumor cells. This allows identification of the most effective individual therapy and enables a personalized treatment tailored specifically to each patient.
-
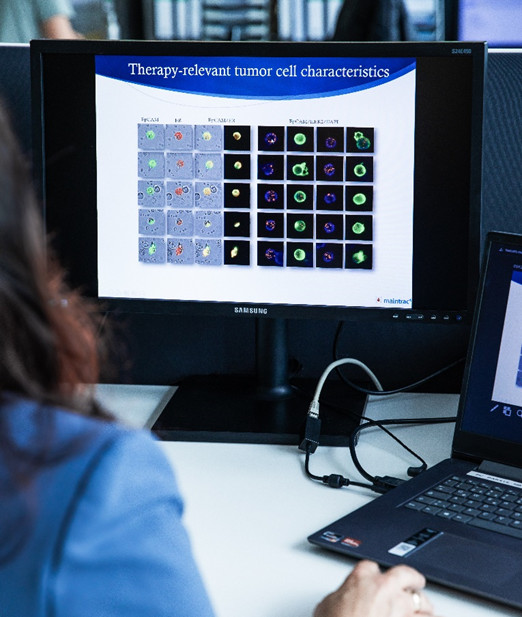
Therapie-relevant Characteristics
maintrac® analysis of therapy-relevant charakteristics enables the identification of specific markers on circulating tumor cells that provide insights into the response to various therapies. This supports personalized therapy planning and helps select the most effective treatment options for the patient.
-
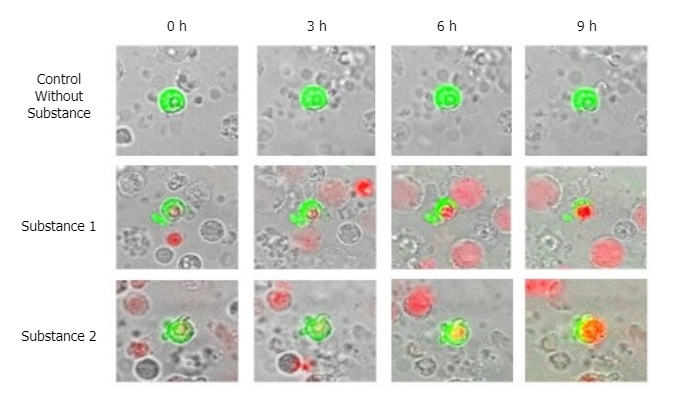
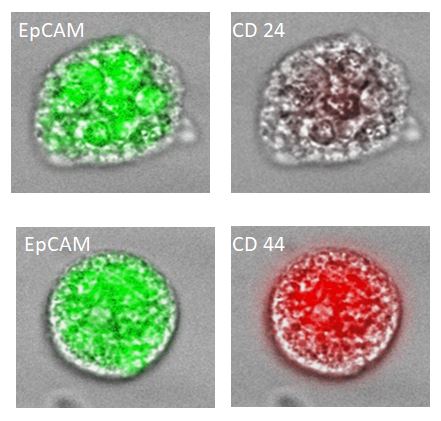
Tumorspheres
stemtrac® tumorspheres is a functional test for the detection of circulating cancer stem cells. The number of tumorspheres is associated with the aggressiveness of the primary tumor and the risk of disease progression. The test helps assess the metastatic potential of the tumor and supports personalized oncology treatment.
-
Timing of the Blood Sample Collection
The timing of the blood draw is crucial for the accuracy of the results. Only precise sample collection at the optimal time can ensure reliable and meaningful results.
-
Cell Counting
- News
- Order
-
Download
-
Download
On this page, you can find out about how to receive the lab request form for maintrac®; you will also be provided with information on how the blood samples should be send.
-
Information material
You can download our current information documents here. There you will learn about the applications of maintrac®.
-
Publications
Obtain an overview Verschaffen Sie sich einen Überblick zu den wichtigsten Studien und Publikationen der letzten Jahre. Bei Fragen zur Literatur wenden Sie sich gerne direkt an uns.
-
Download